Validation of analysis method for determining ketoprofen concentration in pharmaceutical dosage form using high performance liquid chromatography
Abstract
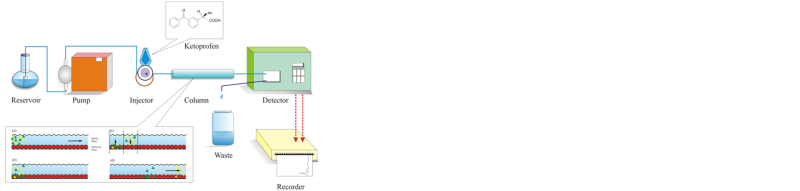
The study was conducted with the purpose to develop and validate a high performance liquid chromatography method with UV detector to determine ketoprofen content in tablet preparation using ethanol-phosphate buffer (pH = 6.0, 80:20, v:v). The method was validated toward parameters of accuracy, precision, linearity, selectivity, LOD and LOQ. The results obtained fulfill the validation requirement of the ketoprofen tablet in the form of LOD = 0.5302 ppm and LOQ = 1.7676 ppm.
Keyword(s)
European Journal of Chemistry, 4 (1), (2013), 58-60
DOI: http://dx.doi.org/10.5155/eurjchem.4.1.58-60.678
References
[1]. Aznan, H. J.; Juli, S. Use of Anti-Inflammatory Non-Steroidal Rational at Tackling Rheumatic Pain. South Sumatera University Publishing, 2004.
[2]. Ferdiansyah, F. Behavior of Ketoprofen Diffusion through the Chitosan-CMC membrane. Bogor Agricultural Institute Publishing, 2009.
PMid:18651245
[3]. Epshtein, N. A. Pharm. Chem. J. 2005, 34(4), 212-228.
[4]. Qomariah, N. The Influence of an Tromethamine Organic amine base to the solubility of ketoprofen, Airlangga University Publishing, 2007.
[5]. Ermer, J. Method Validation in Pharmaceutical Analysis. Weinheim: Wiley-VCH Verlag GmbH & Co. KgaA, 2005.
http://dx.doi.org/10.1002/3527604685
[6]. Brown, P.; Deantonis, K. High Performance Liquid Chromatography, In. F. A., Settel (eds), Handbook or Instrumental Techniques for Analytical Chemistry, Prentice-Hall Inc., 1997.
[7]. Darnpanid, U. The effect of Phospholipid as Penetration Enhancer on Skin Permeation of Ketoprofen, Mahidol University Publishing, 2004.
[8]. Mallikarjuna, N. R.; Kondi, R. K.; Bagyalakshmi, J.; Ravi, T. K.; Ramakotaiah, M. Int. J. Res. Pharm. Sci. 2010, 1(2), 190-194.
[9]. Marcio, F.; Liberato, B. J.; Micheli, W.; Thiago, B.; Sergio, L. D. Acta Farm. 2006, 25 (1), 117-122.
[10]. World Health Organization. Validation of analytical Procedures Used in The examination of Pharmaceutical Materials, WHO Technical Report Series No. 823, 2007.
[11]. United States Pharmacopeia. The National Formulary 30th edition, The United States Pharmacopeia Convention, 1407, 2007.
[12]. Srinivasu, K.; Vankateswara, J. R.; Appala, N. R.; Mukkanti, K. E-J. Chem. 2011, 8(1), 453-456.
Refbacks
- There are currently no refbacks.



























