Synthesis and reactions of 3-aminotetrachloroquinazolin-2,4-dione
Abstract
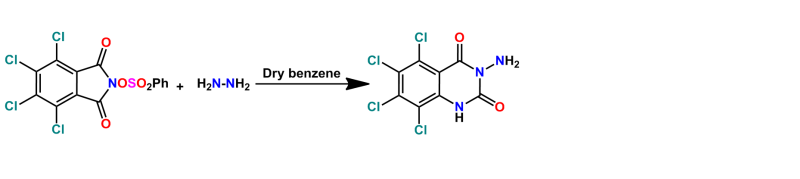
N-phenylsulphonyloxytetrachlorophthalimide was obtained by treatment of N-hydroxy tetrachlorophthalimide with benzenesulphonyl chloride. Also, the titled compound 3 was obtained by reaction of compound 2 with hydrazine hydrate via Lossen rearrangement. Compound 3 used as starting material for the synthesis of new pyrimidine and quinazolinedione derivatives containing four chlorine atoms which have pharmacological activity.
Keyword(s)
European Journal of Chemistry, 2 (4), (2011), 514-518
DOI: http://dx.doi.org/10.5155/eurjchem.2.4.514-518.479
Cited-By
[1]. Synthesis and spectral characterization of some heterocyclic nitrogen compounds
Mamdouh Adly Hassan, Maghrabi Ali Seleem, Ahmed Mohamed Mosallem Younes, Mohamed Mobark Taha, Abou-Bakr Haredi Abdel-Monsef
European Journal of Chemistry Volume: 4 Issue: 2 First page: 121 Year: 2013 
References
[1]. Agrawal, J. P.; Chouk M. P. Res. Ind. J. 1982, 27, 19-21.
[2]. Sou, S.; Mayumi, S.; Takahashi, H.; Yamasaki, R.; Kadoya, S.; Sodeoka, M.; Hashimoto, Y. Bioorg. Med. Chem. Lett. 2000, 10, 1081-1086.
http://dx.doi.org/10.1016/S0960-894X(00)00161-X
[3]. Valencia, R.; Mason M. J.; Woodruff C. R.; Zimmering, S. Environ. Mutagen. 1985, 7, 325-348.
http://dx.doi.org/10.1002/em.2860070309
PMid:3930234
[4]. Wenzel, D. G. J. Am. Pharm. Assoc. 1995, 44, 550-553.
http://dx.doi.org/10.1002/jps.3030440911
[5]. Hansen, P.; Pederesen, E. B. Acta Chem. Scand. 1990, 44, 522-523.
http://dx.doi.org/10.3891/acta.chem.scand.44-0522
[6]. Maillard, J.; Vincent M.; Benard, M. Chem. Ther. 1968, 3, 100-106.
[7]. Hong, E. U. S. Patent 3726979. Chem. Abstract. 1973, 79, 27560a.
[8]. Akgun, H.; Hollstein, U.; Hrwitz L. J. Pharm. Sci. 1988, 77, 735-739.
http://dx.doi.org/10.1002/jps.2600770902
[9]. Havera, H. J.; Vidiro H. J. Med. Chem. 1979, 22, 1548-1550.
http://dx.doi.org/10.1021/jm00198a024
PMid:231656
[10]. Havera H. J. Ger. Patent 242858, (1979). Chem. Abstract 1975, 82, 171029p.
[11]. Fahmy, A. F. M.; Youssef, M. S. K.; Abdel Halim, M. S.; Hassan M. A.; Saur, J. Heterocycles 1986, 24, 2201-2213.
http://dx.doi.org/10.3987/R-1986-08-2201
[12]. Fahmy, A. F. M.; Aly, N. F.; Nada A.; Aly N. Y. Bull. Chem. Soc. Jpn. 1977, 50, 2678-2681.
http://dx.doi.org/10.1246/bcsj.50.2678
[13]. Silverstein, R. M.; Bassler, G. C.; Morrill, T. C. Spectrometric Identification of Organic Compounds, John Wiley, 1916, 35.
[14]. Seeley, H. W.; VanDemank, P. J. Selected Exercises from Microbes in Action A laboratory Manual of Microbiology, 3rd Ed., W.H. Freeman & Co Ltd., 1981.
[15]. Ryan, K. J; Ray, C. G. Sherris Medical Microbiology (4th edn), McGraw Hill. ISBN 0-8385-8529-9, 2004.
[16]. Selassie, C. D.; Li, R.; Poe, M.; Hansch, C. J. Med. Chem. 1991, 34, 46-54.
http://dx.doi.org/10.1021/jm00105a008
[17]. Cheng, C. C. Prog. Med. Chem. 1969, 6, 67-134.
http://dx.doi.org/10.1016/S0079-6468(08)70197-8
[18]. Nair-Scott, D. B. M.; Ulbrich, T. L. V.; Rogers, M. L.; Chu, E.; Rose, C. Cancer Res. 1959, 19, 15-19.
PMid:13638986
[19]. Narla, R. K.; Myres, D. E. Clin. Cancer Res. 1998, 4, 1405-1414.
PMid:9626456
Refbacks
- There are currently no refbacks.



























