Synthesis of new 4,6-disubstituted-1,3-5-triazin-2-yloxy esters and N-hydroxyamides
Abstract
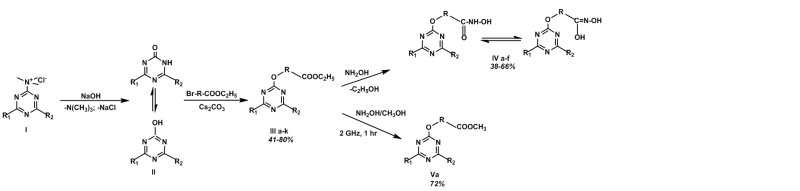
A convenient method for synthesis of new sym-triazine ester and hydroxamate derivatives has been developed. Various reaction conditions were studied and optimized, and a series of new 1,3,5-triazine based esters and N-hydroxyamides were obtained with good yields (38-80%). The reaction between oxo-derivatives of 4,6-disubstituted-1,3,5-triazines and halogenated carboxylic esters derivatives using Cs2CO3 as a catalyst was found to be the most convenient method for 4,6-disubstituted-1,3,5-triazine-2-yloxy esters synthesis. These 1,3,5-triazin based esters served as precursors for the synthesis of 4,6-disubstituted-1,3,5-triazin-2-yloxy-N-hydroxybutanamides using solution of hydroxylamine hydrochloride in dry methanol and KOH at room temperature. Structures of the newly synthesized compounds were obtained by 1H NMR, 13C NMR, MS, IR spectral data and elemental analysis.
Keyword(s)
European Journal of Chemistry, 1 (4), (2010), 302-306
DOI: http://dx.doi.org/10.5155/eurjchem.1.4.302-306.185
References
[1]. Knuniants I. Chem. Encyclopedia, 5th edition, v. 1, Sovietskaya encyclopedia Publishing House, Moscow, 1988.
[2]. Fazary, A. E.; Khalil, M. M.; Fahmy, A.; Tantawy, T. A. Medical Journal of Islamic Academy of Sciences, 2001, 14(3), 109-116.
[3]. Dooley, C. M.; Devocelle, M.; McLoughlin, K. B.; Nolan, K. B.; Fitzgerald, D. J.; Sharkey, C. T. Mol. Pharmacol. 2003, 63(2), 450-455.
doi:10.1124/mol.63.2.450
PMid:12527817
[4]. Nigović, B.; Kujundžić, N.; Sanković, K.; Vikić-Topić, D. Acta Chim. Slov. 2002, 49(3), 525-535.
[5]. Nirmal, K.; Huber, V.; Smith, P.; Gopalan, A. Tetrahedron, 1994, 50(9), 2657-2664.
doi:10.1016/S0040-4020(01)86981-7
[6]. Huang, L.; Pardee, A. B. Mol. Medicine, 2000, 6(10), 849-866.
PMid:11126200 PMCid:1949918
[7]. Taylor, R. Czech. J. Phys. 1999, 49, 617-623.
doi:10.1007/s10582-999-1041-0
[8]. Matijevic, J.; Samarzija, I.; Honovic, L.; Jurisic, B. Acta Pharm. 2008, 58, 231-236.
doi:10.2478/v10007-008-0010-7
PMid:18515233
[9]. Kobashi, K.; Takebe, S.; Terashima, N.; Hase, J. J. Biochem. 1975, 77, 837-843.
[10]. Mishra, H.; Parrill, A. L.; Williamson, J. S. Antimicrob. Agents Chemother. 2002, 46(8), 2613-2618.
doi:10.1128/AAC.46.8.2613-2618.2002
PMid:12121941 PMCid:127352
[11]. Emanuele, S.; Lauricella, M.; Tesoriere, G. Int. J. Oncol. 2008, 33(4), 637-646.
PMid:18813776
[12]. Massaro, A.; Mordini, A.; Reginato, G.; Russo, F.; Taddei M. Synthesis. 2007, 20, 3201-3204.
[13]. Ning, L.; Greenblatt, D. Y.; Kunnimalaiyaan, M.; Chen, H. Oncologist, 2008, 13(2), 98-104.
doi:10.1634/theoncologist.2007-0190
PMid:18305053
[14]. Niemeyer, H. M. Euphytica, 1988, 37(3), 289-293.
[15]. Zasada, I.; Meer, S.; Halbrendt, J.; Rice C. Nematology, 2005, 95, 1116-1121.
[16]. Holland, K. P.; Elford, H. L.; Bracchi, V.; Annis, C. G.; Schuster, S. M.; Chakrabarti, D. Antimicrob. Agents Chemother. 1998, 42(9), 2456-2458.
PMid:9736585 PMCid:105855
[17]. Blotny, G. Tetrahedron, 2006, 62(41), 9507-9522.
doi:10.1016/j.tet.2006.07.039
[18]. Smolin, E.; Rappoport, L. s-Triazine and Derivatives, Interscience, NY, 1959.
[19]. Melnikov, N.; Bascakov, Y. Chemistry of Herbicides and Growth Regulators of Plants, Goskhimizdat Publishing House, Moscow, 1962.
[20]. Pogosian, G.; Pankratov, V.; Zaplishny, V.; Matsoyan, S.; Korshak, V. Polytriazines, Armenian SSR Academy of Sciences Publishing House, Erevan, 1987.
[21]. Katritzky, A.; Rees, C.; Scriven, E.; Potts, K. Comprehensive Heterocyclic Chemistry, v. 5, Pergamon, Oxford, 1984.
[22]. Demirayak, S.; Karaburun, A. C.; Beis R. Eur. J. Med. Chem. 2004, 39(12), 1089-1095.
doi:10.1016/j.ejmech.2004.09.005
PMid:15571871
[23]. Antonian, S.; Zaplishny, V.; Pogosian, G.; Libinzon, R.; Lavretskaya, E.; Pijov, V.; Bessudnova, N.; Sarkisian, D.; Vatolkina O. Khimiko-Farmatsevticheskii Zhurnal 1986, 20(2), 172-175.
[24]. Mikhaylichenko, S.; Dounin, V.; Zaplishny, V. Effectivity of the PASS program in Predicting Biological Activity for sym-Triazine Derivatives. Fourth International Symposium on Computational Methods in Toxicology and Pharmacology Integrating Internet Resources, Moscow, Russia, September 1-5, 2007.
[25]. Mikhaylichenko, S.; Dounin, V.; Zaplishny, V.; Chesnyuk, A. Synthesis and Potential Bioactivity of New Pyrazolyl-sym-Triazine Derivatives. Fourth International Symposium on Computational Methods in Toxicology and Pharmacology Integrating Internet Resources, Moscow, Russia, September 1-5, 2007.
[26]. Chesnyuk, A.; Mikhaylichenko, S.; Konyushkin, L.; Kotlyarov, N.; Zaplishny, V. Patent RU, Chem. Abstr. 2005, 144, 22947.
[27]. Chesnyuk, A.; Mikhaylichenko, S.; Firgang, S.; Kotlyarov, N.; Zaplishny, V. Patent RU, Chem. Abstr. 2006, 144, 345097.
[28]. Chesnyuk, A.; Mikhaylichenko, S.; Konyushkin, L.; Kotlyarov, N.; Zaplishny, V. Patent RU, Chem. Abstr. 2005, 144, 345098.
[29]. Giacomelli, G.; Porcheddu, A.; Salaris, M. Org. Lett. 2003, 5(15), 2715-2717.
doi:10.1021/ol034903j
PMid:12868897
[30]. Bethel, W. J.; Rowayton, R. Patent US 4, 939, 213, Chem. Abstr. 1990.
[31]. Maffezzoni, R.; Zanda, M. Tetrahedron Let. 2008, 49(35), 5129-5132.
doi:10.1016/j.tetlet.2008.06.101
[32]. Anandan, S. K.; Ward, J. S.; Brokx, R. D.; Denny, T.; Bray, M. R.; Patel, D. V.; Xiao, X. Y. Bioorg. Med. Chem. Lett. 2007, 17(21), 5995-5999.
doi:10.1016/j.bmcl.2007.07.050
[33]. Hauser, C. R. Renfrow, W. B. Org. Synth. 1939, 19, 15-17.
[34]. Ho, C. Y.; Strobel, E.; Ralbovsky, J.; Galermo, R. A. J. Org. Chem. 2005, 70(12), 4873-4875.
doi:10.1021/jo050036f
PMid:15932334
[35]. Jones, L. W. and Hurd, C. D. J. Am. Chem. Soc. 1921, 43(11), 2422-2448.
doi:10.1021/ja01444a016
[36]. Chesnyuk, A.; Mikhaylichenko, S.; Zavodnov, V.; Zaplishny, V. Chem. Heterocycl. Compd. 2002, 38(2), 177-182.
doi:10.1023/A:1015335124677
[37]. Mikhaylichenko, S.; Chesnyuk, A.; Zavodnik, V.; Firgang, S.; Koniushkin L.; Zaplishny, V. Chem. Heterocycl. Compd. 2002, 38(3), 292-299.
doi:10.1023/A:1015631203046
[38]. Gordon, A. J.; Ford, R. A. The Chemist’s Companion. Handbook of Practical Data, Techniques, and References, John Wiley&Sons, 1973.
[39]. Jerry, R. M; Christina, N. H.; Paul, F. S. Techniques in Organic Chemistry, W. H. Freeman, 2006.
[40]. Mikhaylichenko, S.; Chesnyuk, A.; Konyushkin, L.; Zaplishny, V. Russ. Chem. Bull. 2003, 52(10), 2157-2160.
doi:10.1023/B:RUCB.0000011872.22319.7e
[41]. Chesnyuk, A.; Mikhaylichenko, S.; Konyushkin, L.; Firgang, S.; Zaplishny, V. Russ. Chem. Bull. 2005, 54(8), 1900-1906.
doi:10.1007/s11172-006-0056-y
[42]. Mikhaylichenko, S.; Chesnyuk, A.; Konyushkin, L.; Zaplishny, V. Chem. Heterocycl. Compd. 2006, 42(5), 642-647.
doi:10.1007/s10593-006-0140-0
[43]. Chesnyuk, A.; Mikhaylichenko, S.; Zaplishny, V.; Konyushkin, L.; Firgang, S.; Chem. Heterocycl. Compd. 2008, 44(3), 339-348.
doi:10.1007/s10593-008-0050-4
[44]. Vogel, A. J.; Tatchell, A. R.; Furnis, B. S.; Hannaford, A. J.; Smith, P. W. G. Vogel’s Textbook of Practical Organic Chemistry, 5th edition, Prentice Hall, 1996.
Refbacks
- There are currently no refbacks.



























