Synthesis and antimicrobial evaluation of some new polyheterocyclic systems containing 1,2,4-triazine moiety
Abstract
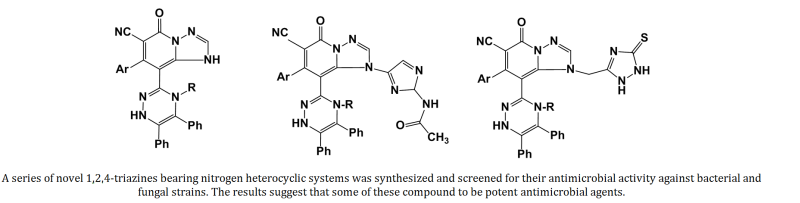
7-(4-Chloro/3-nitrophenyl)-8-[5,6-diphenyl-4-(1,3-thiazol-2-yl)-1,4-dihydro-1,2,4-triazin-3-yl]-5-oxo-1,5-dihydro[1,2,4]triazolo[1,5-a]pyridine-6-carbonitrile (6a, b) was utilized as a key intermediate for the target polyheterocyclic systems. Reactions of 6a, b with halocarbonyl reagents followed by heterocyclization with bi-nitrogen nucleophiles gave some new nitrogen heterocycles (7-13). Structures of the new compounds were established by elemental analyses and spectral data. The synthesized compounds were screened for their antimicrobial activity.
Keyword(s)
European Journal of Chemistry, 1 (3), (2010), 168-172
DOI: http://dx.doi.org/10.5155/eurjchem.1.3.168-172.29
Cited-By
[1]. Synthesis and Antimicrobial Activities of Some 6-Methyl-3-Thioxo-2,3-Dihydro-1,2,4-Triazine Derivatives
Ahmed A. El-Barbary, Ashraf A. El-Shehawy, Nabiha I. Abdo
Phosphorus, Sulfur, and Silicon and the Related Elements Volume: 189 Issue: 3 First page: 400 Year: 2014 
[2]. Voltammetric behavior, biocidal effect and synthesis of some new nanomeric fused cyclic thiosemicarbazones and their mercuric(II) salts
M.S.T. Makki, R.M. Abdel-Rahman, M.S. El-Shahawi
Arabian Journal of Chemistry Year: 2011 
References
[1]. El-Gendy, Z.; Morsy, J. M.; Allimony, H. A; Abdel-Monem Ali, W. R.; Abdel-Rahman, R. M. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178(9), 2055-2071.
doi:10.1080/10426500390228738
[2]. Erickson, J. G. Chem. Heterocycl. Compd. 1956, 10, 44-51.
doi:10.1002/9780470186589.ch2
[3]. Jones, R. L.; Kershaw, J. R. Rev. Pure Appl. Chem. 1971, 21, 23-29.
[4]. Neunhoeffer, H.; Wiley, P. F. Chem. Heterocycl. Compd. 1978, 33, 189-196.
doi:10.1002/9780470187050.ch10
[5]. El Ashry, E. H.; Rashed, M.; Taha, E.; Ramadan, E. Adv. Heterocycl. Chem. 1994, 59, 39-46.
doi:10.1016/S0065-2725(08)60007-0
[6]. Abdel-Rahman, R. M. Pharmazie 2001, 56, 275-281.
PMid:11338663
[7]. El-Gendy, Z.; Morsy, J. M.; Allimony, H. M.; Abdel-Monem Ali, W. R.; Abdel-Rahman, R. M. Pharmazie 2001, 56, 376-381.
PMid:11400552
[8]. Hunt, J. T.; Mitt, T.; Borzilleri, R.; Brown, J.; Fink, B.; Bhide. J. Med. Chem. 2007, 47, 4054-4059.
doi:10.1021/jm049892u
PMid:15267243
[9]. Sztanke, K.; Rzmowska, J.; Niemczyk, M.; Dybala, I.; Koizoi, A. Eur. Med. Chem. 2006, 41, 1373-1379.
doi:10.1016/j.ejmech.2006.06.013
PMid:16996655
[10]. Sarvesh Kumar, P.; Abhishek, F.; Ashutash, S.; Nizamuddin, S. J. Eur. Med. Chem. 2009, 44, 1188-1196.
doi:10.1016/j.ejmech.2008.05.033
PMid:18614258
[11]. Deeb, A.; El-Mariah, F.; Hosny, M. A. Bioorg. Med. Chem. Lett. 2004, 14, 5013-5017.
doi:10.1016/j.bmcl.2004.06.102
[12]. Cai, S.; Li, Q. S.; Borchardt, R. T.; Kuczera, K.; Schowen, R. L. Bioorg. Med. Chem. 2007, 15, 7281-7287.
doi:10.1016/j.bmc.2007.08.029
[13]. Demirbas, A.; Ceylan, S.; Demirbas, N. J. Heterocycl. Chem. 2007, 44, 1271-1280.
doi:10.1002/jhet.5570440608
[14]. Gewald, K. G.; Schnbelt, A.; Martin, G. J. Prakt. Chem. 1975, 317, 561-569.
doi:10.1002/prac.19753170407
[15]. Al-Omran, F.; Abdel Khalik M.; Elna, M. H. J. Heterocycl. Chem. 1995, 6, 545-564.
[16]. Abdel-Monem, W. R. Chem. Pap. 2004, 276-282.
[17]. Abdel-Monem, W. R. Boll. Chim. Farmaco, 2004, 143(6), 239-247.
PMid:15881802
[18]. Abdel-Monem, W. R. Boll. Chim. Farmaco, 2005, 144(5), 1-23.
[19]. Abdel-Monem, W. R.; Ali, T. E. Int. J. Chem., 2007, 17, 303-314.
[20]. Al-Najjar, A. A. A.; Amer, S. A.; Riad, M.; Elghamy, I.; M. H. Elnagdy, M. H. J. Chem. Research (S), 1996, 296-297.
[21]. Gould, J. C.; Bowi, J. M. J. Eur. Med. Chem.1952, 59, 198-205.
[22]. Singh, A.; Latita, R.; R. Dhakareg, R.; Saxena, G. C. J. Indian Chem. Soc. 1996, 73, 339-348.
[23]. Chaudhaxy, A.; Singh, R. V. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178, 603-609.
doi:10.1080/10426500307927
[24]. Saraswati, S.; Utpal, D.; Parikh, A. R. J. Indian Chem. Soc., 1994, 71, 159-167.
Refbacks
- There are currently no refbacks.



























